SIG together with Resistomap – Finland, Establish Biotechnology Collaboration in Testing of Antimicrobial Resistant (AMR)


Resistomap Oy and PT Saraswanti Indo Genetech (SIG) agreed to build cooperation by signing a Antimicrobial Resistance Monitoring Service Business Agreement. The signing took place at the Embassy of Finland, Jl. Mega Kuningan Timur I No.E.1,2, Jakarta Selatan, on Friday, 9 December 2022. This Business Agreement was signed by the CEO of Resistomap, Ms. Windi Muziasari and the President Director of SIG, Mr. M Edi Premono. It is a form of mutual commitment in complimenting each other`s duties and function in the perspective of Biotechnology, particularly in laboratorial services in Indonesia with the support from Finnpartnership, the Ministry of Foreign Affair of Finland, the Embassy of Finland in Jakarta, Indonesia, the Ministry of Foreign Affair of Indonesia, and the Embassy of Indonesia in Helsinki, Finland. This collaboration is facilitated by StepNesia (PT Akselerasi Inovasi Bangsa), an Indonesian based startup consultant.

The Senior Commercial Advisor, on behalf of the Ambassador of Finland to Indonesia, Ms. Nina Jacoby welcomed this cooperation as a positive sign and progress toward the relationship between Indonesia and Finland. “It is a great opportunity to see Finnish-Indonesia cooperation, especially for high-level tech companies. The two countries have a clear aim to develop a higher standard, toward sharing knowledge and operational activities in the environmental and health issues, as well as technological advancement. It is hoped to be a role model for many countries in solving the SDGs issues. We would like to invite more startups and companies to create more collaboration in the future.”

Lucas Schoonman, the team leader for Food and Agriculture Organization (FAO) -Emergency Centre for Transboundary Animal Diseases (ECTAD) Program in Indonesia also emphasizes the important of this business agreement in speeding up the testing for antimicrobial resistance (AMR) in Indonesia. “AMR is also called a ‘silence pandemic’, as 10 million people are estimated to die in to 2050 because of this. Considering the high prevalent of AMR in the environment, we have to take more preemptive action, not only for human health, animal health, but also environmental health. This partnership could help in increasing te laboratory capacity in providing surveillance to monitor the antibiotic resistance gene in the environment”.

“I am very happy that we have this cooperation. I am als hopeful that this cooperation can benefit all of us, both the Indonesia and Finland. especially as the Resistomap has also collaborate academically with UGM. I hope it will also be replicate by other universities [to collaborate with business in doing research]” said Winardi H. Lucky, Director, Directorate of Europe-2, Ministry of Foreign Affair of Indonesia.

Resistomap together with SIG, have a unique knowledge on the combination of microbial antibiotic resistance, molecular genetics and data science. Their mission is to detect and quantify antibiotic resistance genes from environmental samples fast and comprehensively, by using state-of-the-art technology in gene profiling. Thus, in order to achieve that, the existence of a credible analytical test laboratory is an absolute must to create a product that must meet certain prerequisites. The Business Agreement coverage is focused on satellite laboratory services and operation, as well for the improvement of human resources, quality and standards of laboratory services. It is in line with the World Antimicrobial Awareness Week (WAAW) 2022, a global campaign that is celebrated annually to improve awareness and understanding of Antimicrobial resistance (AMR) and encourage best practices among the public to Prevent Antimicrobial Resistance.
Library of Knowledge

Peran Sertifikat Hasil Uji Lab Dalam Perizinan Produk BPOM
Mengurus Perizinan Produk BPOM adalah salah satu langkah penting untuk memastikan keamanan dan kualitas produk yang akan beredar di pasaran. Namun,...
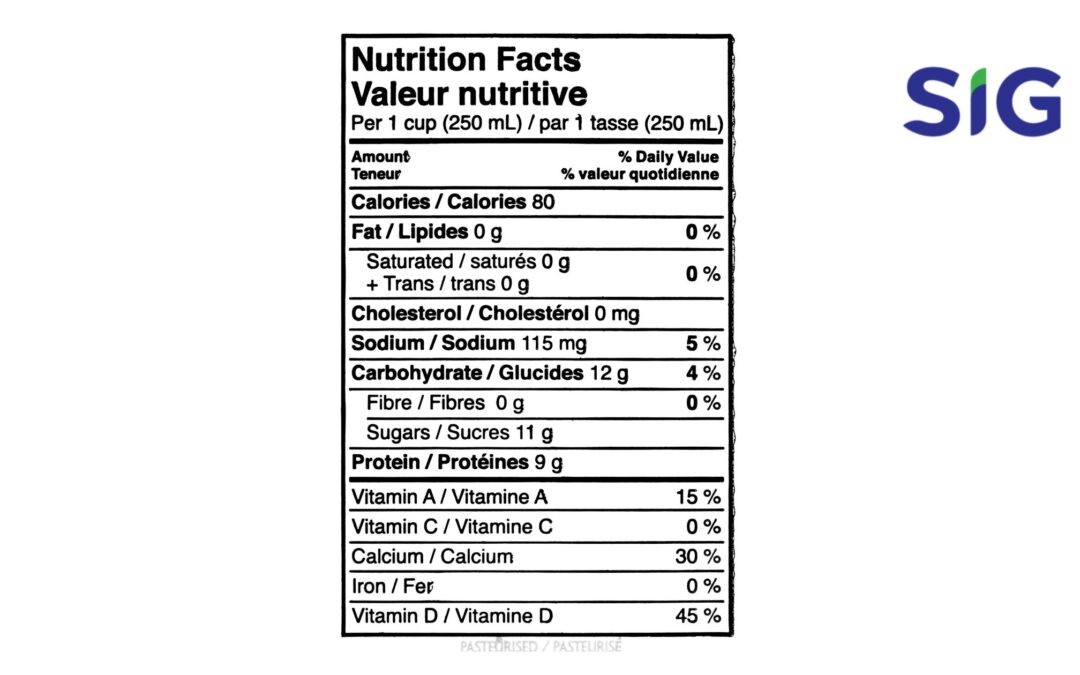
Lakukan Uji Lab di SIG Dan Gunakan Tabel Nutrisinya Untuk Registrasi Produk BPOM
Melakukan registrasi produk BPOM bukanlah hal yang bisa dianggap enteng. Proses ini penting demi memastikan bahwa produk yang akan dipasarkan telah...

Mari Kenali Macam-Macam Persyaratan Registrasi Produk BPOM
Jika kamu sedang merintis bisnis produk makanan, obat, atau kosmetik, kamu pasti sudah tidak asing dengan istilah BPOM. BPOM atau Badan Pengawas...

Yuk Cari Tahu Prosedur Registrasi Produk BPOM Hingga Terbit Izin Edar
Prosedur registrasi produk BPOM menjadi langkah penting bagi setiap pelaku usaha yang ingin menjual produknya di Indonesia. Tanpa izin dari BPOM,...
SIG Laboratory
Graha SIG, Jl Rasamala No. 20, Taman Yasmin, Bogor, Jawa Barat 16113.
Phone. +62 251 7532 348
WhatsApp. +62 82 111 516 516
Email. marketing-sig@saraswanti.com
SIG Jakarta
Jl. Percetakan Negara No. 52 B RT 006 / RW 001, Rawasari, Cempaka Putih, Jakarta Pusat 10570.
Phone. +62 21 2147 9292
SIG Surabaya
AMG Tower, 12th Floor, Jl. Dukuh Menanggal 1-A, Gayungan, Surabaya, Jawa Timur, 60234.
Phone. +62 31 8253 1288
WhatsApp. +62 818 885 165
Email. marketing@sigsurabaya.com
SIG Semarang
Jl. Kanfer Raya Blok R No. 4 Pedalangan, Kec. Banyumanik, Kota Semarang, Jawa Tengah 50268.
Phone. +62 24 7004 0541
WhatsApp. +62 812 9000 5165
Email. cs.sigsmg@saraswanti.com
SIG Medan
Jl. Bunga Asoka, Ruko Komp. Asoka Raya Residance No. 1, Medan Selayang, Sumatera Utara 20133,
WhatsApp. +62 822 7207 9665
Email. salesmedan.sig@saraswanti.com
SIG Yogyakarta
WhatsApp. +62 896 4856 9422
Email. arifin.sig@saraswanti.com
SIG Makassar
WhatsApp. +62 853 3843 9816
Email. anwar@sigsurabaya.com
Operational Hours
Monday to Friday
08.00 - 17.00 WIB.


